In agriculture, most growers think of sodium (Na) as an unwanted intruder when, in fact, it is an essential mineral for thriving and healthy plant growth in halophytes and C4 plants. Sodium is a crucial electrolyte that helps facilitate the thousands of messaging reactions that are part of the electrical life of the plant. It is not an essential mineral, but it can be used in small amounts to open stomata and chlorophyll formation in plants. Therefore, it is all the more necessary to identify the right amounts of sodium present in your plot of land so that your crops get adequate nutrition.
With the help of a digital soil tester in the USA, you can quickly establish the amount of sodium and take the necessary steps if the present amounts are not adequate.

Understanding ‘sodic soils’:
Soils with high exchangeable sodium (Na) levels and low levels of total salts are called the sodic soils. Sodic soils may affect plant growth by:
1.) Specific toxicity to sodium-sensitive plants;
2.) Nutrient deficiencies or the imbalances;
3.) High pH;
4.) Dispersion of the soil particles that causes poor soil physical condition.
Sodic soils tend to develop a poor structure and drainage over time because the sodium ions on clay particles cause soil particles to deflocculate or even disperse. Sodic soils are very hard and cloddy when dry and tend to crust. The water intake is usually poor with sodic soils, especially those that are high in silt and clay. Poorer plant growth and germination are also expected. The soil’s pH is generally high, often above 9.0, and plant nutritional imbalances may occur. A soil pH over 8.4 typically indicates that a sodium problem actually exists. The terminology “alkali” is often used to describe soils high in salt, but sometimes people use the term to mean high pH and, at other times, high sodium. “Black alkali” refers to the condition of sodic soil where organic matter has spread and is a dusty material on the soil surface.
Salinity:
Salinity measures the salt concentration in soil. Saline soils are uncommon in Minnesota but may occur if a specific salt source exists (e.g., road salt, biosolids, or manure application), in irrigated soils, and in arid soils.
Electrical conductivity or EC is a good indicator of soil salinity. EC values greater than 16 dS/m indicate saline soils. If EC suggests saline soils, taking laboratory samples to identify specific chemicals of concern (e.g., sodium, potassium) may be helpful.
Sodium Adsorption Ratio (SAR)
Sodium levels in soil layer are often reported as Sodium Adsorption Ratio (SAR). This is a ratio of the quantity of cationic (positive) charge contributed to the soil by sodium to that contributed by concentrations of Calcium (Ca) and Magnesium (Mg). The SAR is identified from a water extract of a saturated soil paste. The soil is classified as Sodic if the SAR is above 13 (Table 1).
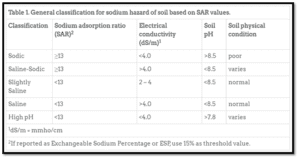
However, sodium can cause the deterioration of soil structure and water infiltration problems at SAR levels under 13 in some cases. The severity of the symptoms with high SAR soils depends upon different site-specific factors, including the soil type, texture, drainage conditions and the irrigation water quality. Some soil test reports depict high sodium levels as ESP (exchangeable sodium percentage). An ESP of more than 15% is sometimes used to classify soil as sodic. This means sodium occupies more than 15% of the soil’s Cation Exchange Capacity (CEC). Be aware that sensitive plants may also show injury or poor growth at even lower sodium levels.
What Should Be the Ideal Levels of Sodium?
We should always try to keep sodium below 50 ppm. In terms of base saturation percentages, sodium should never exceed 1.5%. The most important consideration here, however, is to ensure that sodium never exceeds potassium in terms of base saturation percentages. The ideal potassium-to-sodium ratio is five parts potassium to 1 part sodium.
However, an interesting phenomenon occurs when the percentage of sodium ions attached to the clay exceeds the potassium percentage. In this instance, the plant becomes confused. Sodium and potassium are similar-sized ions, and for millions of years in nature, there has always been a more significant percentage of potassium saturating the clay colloid than sodium. However, we have messed up that equation in many areas, and the plant needs to adapt to our mismanagement. When the plant requires potassium, it selects the most abundant lookalikes from the clay. If sodium is more abundant than potassium, that is the mineral uptake. Our crop unintentionally absorbs an unwanted, unproductive mineral rather than potassium (“the money mineral”). Sugars are not moved, fruit and seed do not grow, and yield and profit will be less.
Managing Sodic Soils:
There are usually 3 options for rectifying soil-related problems:
- change the plant species to a much more tolerant species, or,
- change the soil type.
Often, altering the soil is the most difficult of these options.
When soils are high in sodium content, the goal is to replace sodium with calcium concentrations and then leach out the sodium. There are two possible approaches to doing this:
- dissolve limestone (calcium carbonate) or gypsum (calcium sulphate) already existing in the soil or
- add calcium to soil.
If free lime exists in the soil, it can be dissolved by applying sulphur or sulfuric acid. Sulfur products decrease the pH, which dissolves the lime, thus freeing up the calcium. Add calcium if free lime or gypsum is not present in correct amounts as determined by a soil test.
The most common form of calcium used for this is gypsum. Although calcium chloride reacts more quickly and can also be used, it is usually more expensive. After broadcasting the source of calcium on the soil surface, mix it and ensure adequate moisture is present to dissolve it.
Ensure drainage is adequate before amending the soil, and after applying a sulphur product or a calcium source, leach out the sodium with good, quality water. Success in reclaiming the non-irrigated sodic or saline-sodic soils with adequate gypsum application may be possible on coarse-textured soils that receive precipitation over soil water-holding capacity.
If you want to identify the adequate amount of sodium or other minerals in soil, contact our soil testing experts at SoilOptix® now!
