Farming involves a wide variety of knowledge and skills to cultivate a profitable crop successfully. Farmers need to know about the plants they grow, weather, machinery, and the ground in which their crops are grown, such as the pH level of the soil and the nutrients it contains.
Soil pH level is the degree of acidity or alkalinity of a particular plot of land which is determined by the concentration of the hydrogen ions (H+) in the soil. A digital soil pH tester in the USA helps to decide on its existent quantities. Soil pH determination can indicate whether the soil is suitable for the plants or needs to be adjusted to produce optimum plant growth.
Soil pH – the Measure of Soil Acidity:
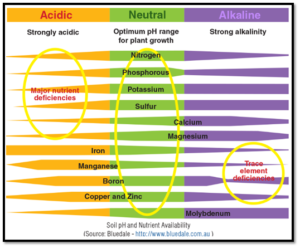
Soil pH measures acidity or alkalinity. The pH scale ranges right from 0 to 14, with pH 7 being neutral. A pH value less than 7 indicates acidic soil, while a pH value greater than 7 indicates alkaline or basic soil. Since pH is measured based on a logarithmic scale, a pH of value 6 is ten times more acidic compared to a pH level of 7.
Soil pH can be evaluated in water (pHw) or in calcium chloride (pHCa), and it will vary depending on the method used. Generally, the pH measured in calcium chloride is 0.7, a pH unit lower than the pH measured in water. Soils become acidic when essential elements like calcium, magnesium, sodium, and potassium held by the soil colloids are replaced by the hydrogen ions. Soils formed under the conditions of high annual rainfall are more acidic than soils formed under more arid conditions.
Soil pH value is considered one of the most vital factors that may determine crop yield. Soil pH can regulate and control many chemical and biochemical reactions within the soil. If the soil pH is too high or too low, it can obstruct nutrient absorption, reduce growth, and even lead to death of plants. Thus, it is essential to maintain an optimal pH level in soil for healthy plant growth.
How Does Soil Acidity Affect Plant Growth and Health?
Acidity in itself is not responsible for restricting plant growth. Instead, biological processes favorable to plant growth can be negatively affected by acidity. Soil pH also impacts the activity of soil microorganisms. The population of bacteria that decompose organic matter declines, and their activity is hindered in highly acidic soil, which results in the accumulation of organic matter and bound nutrients, particularly nitrogen. Soil acidity has the following effects:
- It decreases the availability of essential plant nutrients like phosphorus and molybdenum. It increases the availability of some elements to toxic levels, particularly aluminum and manganese.
- The essential plant nutrients can be leached below the rooting zone.
- Acidity can also degrade the favorable environment for bacteria, earthworms, and other soil organisms.
- Highly acidic soils can also inhibit the survival of valuable bacteria, such as the rhizobia bacteria that fix nitrogen for legumes.
Factors Affecting Soil pH:
Rainfall:
Rainfall contributes to a soil layer’s acidity. Water (H₂O) combines with the carbon dioxide (CO₂) to form a very weak acid — carbonic acid (H₂CO₃). The weak acid ionizes releasing the hydrogen (H⁺) and bicarbonate (HCO₃). The released hydrogen ions then replace the calcium ions held by the soil colloids, causing the soil to become acidic.
The displaced calcium (Ca⁺⁺) ions thereafter combine with the bicarbonate ions to form – calcium bicarbonate soluble and leached from the soil. The net effect is increased soil acidity.
Nitrogen Fertilizers:
Nitrogen levels affect soil pH. Nitrogen sources—fertilizers, manures, legumes—contain or form ammonium, which increases soil acidity unless the plant absorbs the ammonium ions directly. The higher is the nitrogen fertilization rate, the greater will be the soil acidification. As ammonium is transformed into nitrate in the soil (nitrification), H ions are released.
For each pound of nitrogen as ammonium, approximately 1.8 pounds of pure calcium carbonate is needed to neutralize the residual acidity. The nitrate provided or formed can combine with the basic cations like calcium, magnesium, and potassium and leach from the topsoil layer into the subsoil. As these bases are replaced by H ions, soil becomes more acidic.
Plants:
Legumes like soybeans, alfalfa, and clovers take up more cations than anions. This causes the H ions to be released from the plant roots to maintain the electro-chemical balance within their own tissues. The result is net soil acidification.
Subsoil Acidity:
The subsoil may be highly acidic even if the top 6 inches of soil depict a pH above 6.0. When subsoil pH drops below the 5.0-mark, aluminum and manganese in soil become much more soluble and, in some, may be toxic to plant growth. Cotton and soya beans to some extent are a few examples of crops sensitive to highly soluble aluminum levels in the subsoil, and the yields may be reduced under the conditions of low subsoil pH levels.
If you’ve observed areas of stunted plants in your farm, take a subsoil sample in these areas. If the soil pH is highly acidic (below 5.2), lime should be applied as early as in the fall and turned as profoundly as possible.
Liming:
Correcting soil acidity using lime is the foundation of a good soil fertility program. But lime does more than just correct soil acidity. It also:
- Supplies essential plant nutrients, Ca and Mg, if dolomitic lime is used
- It makes other essential nutrients more available
- It prevents elements such as Mn and Al from being toxic to plant growth.
How Do We Measure Soil pH?
Soil pH can be very well measured using a pH meter, by sending soil samples to a soil testing laboratory for analysis, or by utilizing high resolution soil mapping services. These options typically involve chemical testing using specialized equipment, or by hiring a service provider to come map your field with their specialized equipment. In most instances, utilizing a combination of soil sampling techniques along with sensor technologies can provide a more in-depth look at not just pH, but other important soil properties as well. Regardless of which method of analysis you choose to adopt, a digital soil pH tester in the USA can help determine the accurate amount of pH present in your plot, resulting in better management of the soil and growth of your crop.
If you need help determining the acidity levels of your soil layer, contact us at https://soiloptix.com/now!
